The Complexity of SO₂ Measurement in Red Wines
Introduction
There is an un-written rule in winemaking, a rule that influences all our decisions and is so pervasive that it is seldom talked about, realised or even thought of. The rule is the difference in SO₂ for red wines compared to white wines. As winemakers we have much more confidence in our SO₂ levels in white wines, we don’t give them a second thought, but in red wines it’s a totally different story. If I had barrels of Sauvignon Blanc maturing for a year I wouldn’t even plate them for Brett. But I know if I did the same for some reds in barrel for a year, it’s going to end badly.
This is evident in the wines we taste as well, while I have had the occasional bretty Chardonnay, what are the wine types most susceptible to Brett spoilage? What are the wines you always open and sigh at the first sip? 95% of the time it will be a red wine. The reason why is sometimes passed off as red wines are more commonly matured in barrel or red wines have a higher pH and therefore the molecular SO₂ level is lower. While these are both true and contributing reasons, the largest reason for the inadequacy of SO₂ in red wines is anthocyanin-bisulphite complexes and therefore the over-estimation of FSO₂ by the Rankine method. Let’s start at the very beginning of the story.
Bisulphite Complexes
Way back in 1975 Burroughs published a study titled “Determining Free Sulfur Dioxide in Red Wine’ (Burroughs, 1975) which used research from 1967 on anthocyanins and SO₂. They postulated and then discovered that the reaction of bisulphite ions with anthocyanins is extremely rapid and the decomposition equally as rapid. When SO₂ is removed from the system the equilibrium is restored almost instantly by the decomposition of the the bisulphite-anthocyanin bond. The reaction is also influenced by pH, when wine samples are acidified down to a pH of 1-2 the affinity between anthocyanins and bisulphite is greatly reduced.
Taken together, both those factors point towards measurements of FSO₂ that change the wines pH or involve the removal of SO₂ from the equilibrium will show falsely high results if the wine contains anthocyanins I.E. Red Wines. This is exactly what happens in the Rankine method, still employed in every winery in the world today, wine samples are acidified with orthophosphoric acid down to a pH of near 1 and SO₂ is removed via aspiration to react with H₂O₂ in the bubbler flask.
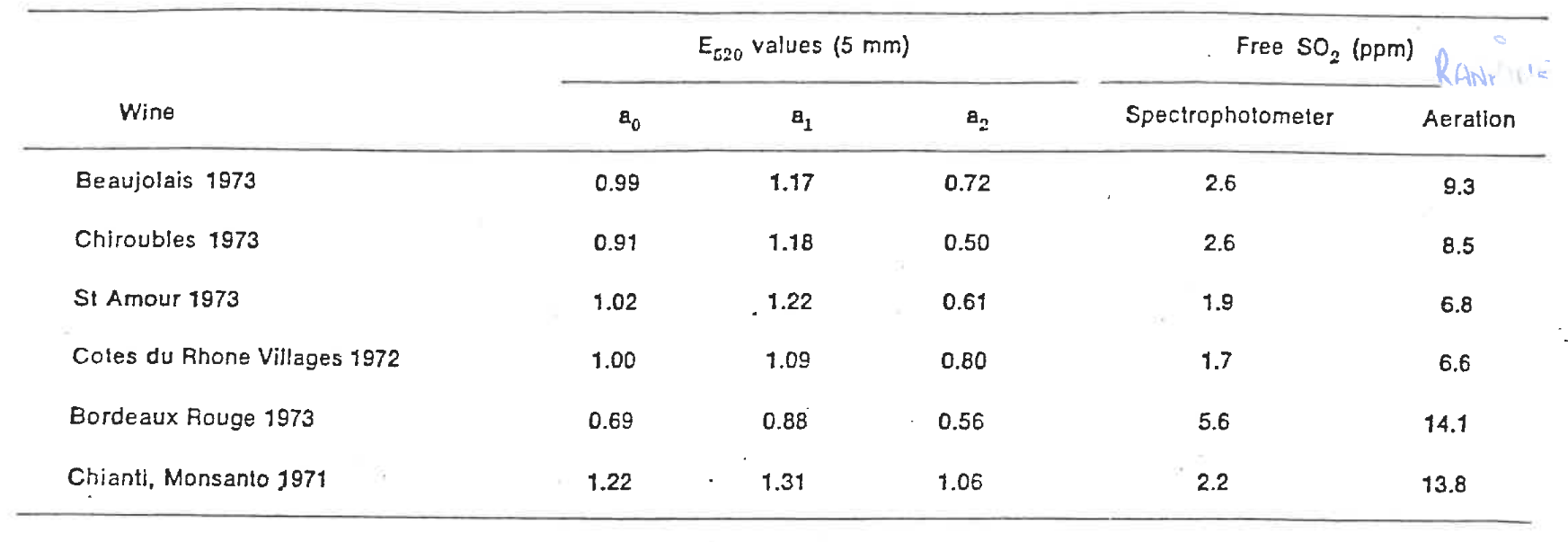
Burroughs confirmed this false result with a method whereby anthocyanins were decolourised by large additions of SO₂ which was compared to the discoloration at normal SO₂ levels. The difference showed the bisulphite decolourisation percentage which was then used to determine the true FSO2 amounts, the resulting values are shown in the table below. We can see the large over-estimation of FSO2 by the conventional aspiration (Rankine) method. This method, however, was not practical for winery laboratory use and was never picked up by industry.
Headspace Detection Methods
Over the years there were a few new methods for the determination of FSO2 such as Electrophoresis (Collins & Boulton, 1996). This study again showed the over-estimation for FSO2 by the Rankine method in red wines. The inability for SO₂ in red wine to control Saccharomyces was also discussed in a 1996 masters thesis out of UC Davis (Bogren, 1996). However the literature continued to utilise disruptive methods for FSO2 determination which led to a large difference in reported molecular SO₂ levels for the control of microbial growth. For instance it might take 0.2 MSO2 to control some brett strains in wine-like mediums but other studies conducted in red wines might see growth above an ‘apparent’ MSO2 of 1, this is further elaborated in Howe et al., (2018).
Pegram et al. (2013) introduced a new method for the detection of FSO2 in wine, namely headspace gas detection tubes which are commonly used in mining. This first approach was just a proof of concept and utilised a pH lowering step to volatilise SO₂, however at the end of the paper they mention in the conclusion:
“A final and intriguing possibility is that the tubes could be used to directly measure headspace SO₂ concentrations, which could then be related to molecular SO₂ concentration in solution via Henry’s Law. Potentially, direct headspace measurement by gas detection tubes could be used for tanks or barrels without the need for drawing a liquid sample, and would also avoid artifacts resulting from dissolution of weak SO₂ adducts due to sample dilution or pH adjustment.”
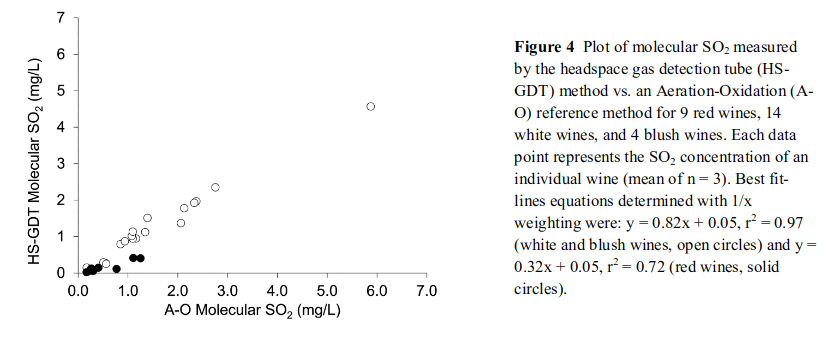
This was exactly the research then performed by Coelho et al., in (2015). Here the authors extended Pegram’s work in 2013 and utilised headspace gas detection tubes, but instead of volatilising the SO₂ in the sample they simply let the headspace equilibrate and then measured the SO₂ in the headspace. This was directly inferred SO₂ in the wine through Henry’s Law. The authors confirmed the results via aspiration with white wines and they conferred well. However in red wines they did not, this is shown in the below figure. The white dots are white wines and show the A-O and HS-GDT methods conferring(r^2 = 0.97) whereas the black dots are red wines where the relationship is not well supported (r^2 = 0.72)
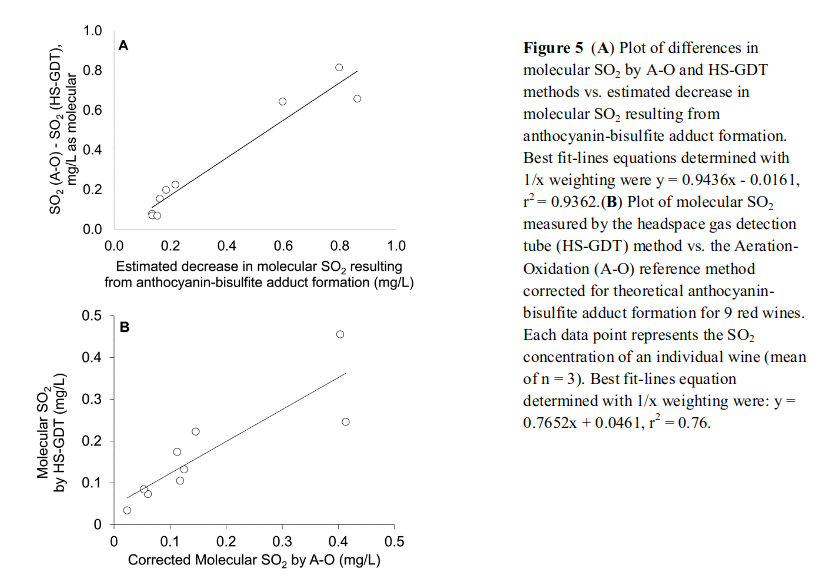
The authors then confirmed this difference to match well with predicted anthocyanin-bisulphite adduct formation. This supports all the previous described work on the issue showing dissociation of anthocyanin-bisulphite adducts during A-O (Rankine) leads to an artificially high reported FSO₂ concentration. The correlation is shown below and I encourage the interested reader to read Coelho et al. (2015) in full as it is extremely interesting.
Anti-Microbial Activity
However the large question remains, when SO₂ is weakly bound to anthocyanins in these adducts does it confer any anti-microbial and/or antioxidant protection? In this post I’ll focus on the anti-microbial perspective and leave the antioxidant question for a follow up article.
To put the problem in another way, does FSO₂ as measured by Rankine or HS-GDT correlate better to anti-microbial activity in red wine? This was exactly the question hypothesised and answered in Howe et al., (2018). This study essentially answers the questions raised at the start of this post around Brett prevention in red wines. The authors used a commercial preservative free white wine sample that they spiked with anthocyanins to simulate a ‘red wine’. They then added EC118 yeast and SO₂ at varying concentrations while measuring cell log reduction as compared to a SO₂ free sample. They made pairs of both white and red wines at differing MSO2 concentrations as measured by HS-GDT and as measured by A-O (Rankine). The results are in the below figure:
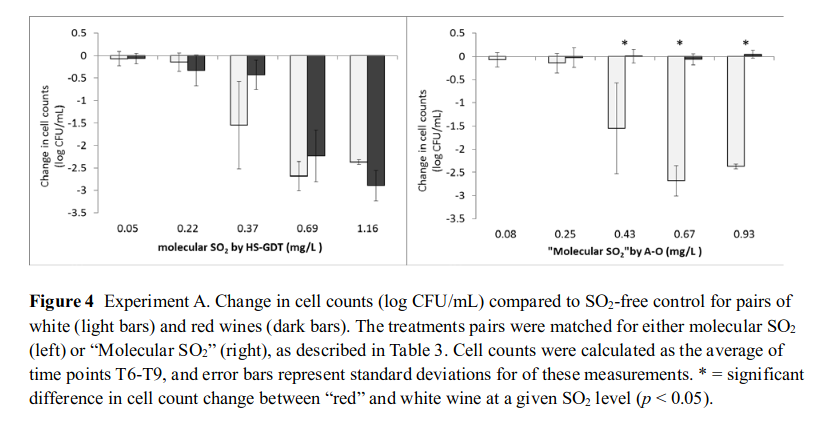
This figure does a little to get your head around, but once you see it, all of the aforementioned studies and science clicks into place. On the left hand side we have the pairs of red/white wines with MSO₂ as measured by HS-GDT and we see comparable log reduction numbers. On the right we have the MSO₂ pairs as measured by A-O and the red bars show basically no cell reduction while in the white wines there is. There is more comprehensive data in the paper by Howe et al. (2018) but we’ll leave it there, essentially this is showing the anthocyanin-bisulphite complexes have negligible antimicrobial activity and thus the conventional A-O (Rankine) method is unsuitable to measure SO₂ anti-microbial activity in red wines. Thus we have our Brett problem, we might believe looking at our SO₂ numbers we have a MSO₂ of 0.8 mg/L, but in reality it could be almost 0 or 0.5 mg/L. How would you ever know?
The Practical Implications
So many practical considerations remain when we think about this. Is FSO₂ as measured by A-O related to SO₂ as measured by HS-GDT? How would a winemaker practically understand their MSO₂ numbers? What is the actual MSO₂ level needed to control Brett? Most studies either don’t even report how they attained their SO₂ number or have used A-O, throwing their numbers into question. I will look into some of these questions in upcoming posts.
References
Bogren, L. N. H. (1996). The effect of sulfur dioxide on Saccharaomyces cereviseae. University of California, Davis.
Burroughs, L. F. (1975). Determining Free Sulfur Dioxide in Red Wine. American Journal of Enology and Viticulture, 26(1), 25–29.
Coelho, J. M., Howe, P. A., & Sacks, G. L. (2015). A Headspace Gas Detection Tube Method for Measurement of SO₂ in Wine without Disruption of Sulfur Dioxide Equilibria. American Journal of Enology and Viticulture. https://doi.org/10.5344/ajev.2015.14125
Collins, T., & Boulton, R. B. (1996). The analysis of free sulfur dioxide and sugars in juices and wines by capillary electrophoresis. Oenologie 95: 5e. Symposium International d’Oenologie, 637–640.
Howe, P. A., Worobo, R., & Sacks, G. L. (2018). Conventional Measurements of Sulfur Dioxide (SO₂) in Red Wine Overestimate SO₂ Antimicrobial Activity. American Journal of Enology and Viticulture, 69(3), 210–220. https://doi.org/10.5344/ajev.2018.17037
Pegram, Z., Kwasniewski, M. T., & Sacks, G. L. (2013). Simplified Method for Free SO₂ Measurement Using Gas Detection Tubes. American Journal of Enology and Viticulture, 64(3), 405–410. https://doi.org/10.5344/ajev.2013.13003